Many brands claim their water filters are "medical-grade," but few explain what that means. This vague marketing puts vulnerable users at risk. Here is the engineering reality behind true clinical safety.
A medical-grade water purifier must achieve specific "Log Reduction1" rates for pathogens, use a sterile pathway resistant to biofilm (typically stainless steel), prevent re-contamination through closed loops, and possess certified validation. It goes beyond simple filtration to ensure absolute biological safety for immunocompromised individuals.

You might think a standard kitchen filter is enough for everyone. However, the difference lies in the microscopic details of the manufacturing and materials. Let’s look at the Engineering Standards2 that separate common appliances from true medical devices.
Plastic piping is the safest material for long-term medical water storage.False
Plastic surfaces are porous on a microscopic level, creating a breeding ground for biofilm and bacteria.
Medical-grade purification requires independent third-party validation.True
Self-testing is not enough; clinical standards require certified validation from bodies like NSF or WQA.
1. Introduction: The Gap Between "Potable" and "Medical-Grade"?
We often confuse safe drinking water with sterile water. The difference is massive. For a healthy adult, tap water is fine. For a patient, it can be dangerous.
Potable water simply meets municipal standards for the general population, allowing for small amounts of bacteria. Medical-Grade Water3 requires the total elimination of opportunistic pathogens that could harm vulnerable immune systems, demanding much tighter engineering tolerances and superior materials.
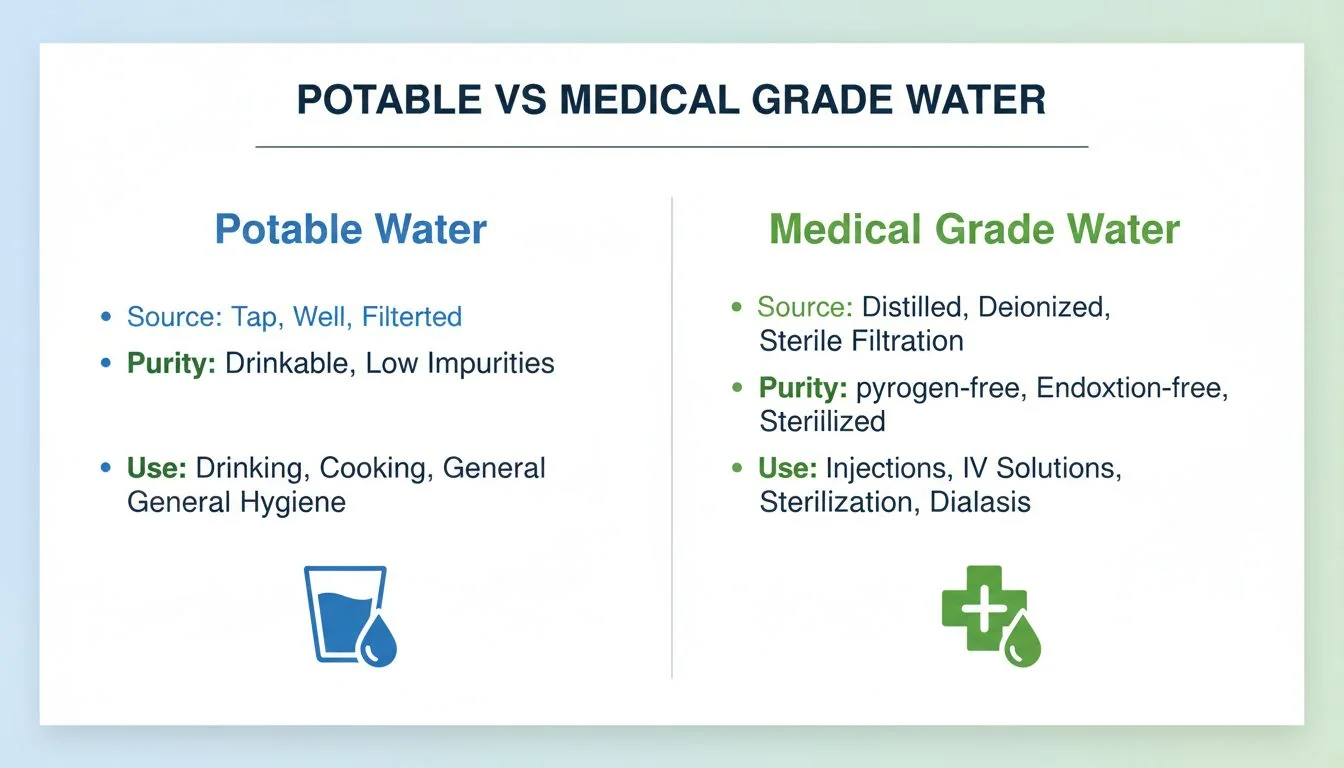
In the manufacturing world, we understand tolerances. If a mold is off by 0.1mm, the part might still fit, but it is not precision engineering. The same logic applies to water. "Potable" water is like a standard molded part—it works for most people. It is safe enough. However, "Medical-Grade" is like a high-precision aerospace component. There is no room for error.
The gap here is biological. Standard filters remove bad taste and some chemicals. Medical-grade systems must remove biological threats. This is critical for the "vulnerable" niche. I am talking about parents mixing formula for infants or families caring for immunocompromised relatives. These buyers are not looking for a cheap appliance. They are looking for safety.
When we design products for this sector, we must shift our mindset. We are not just building a machine that dispenses water. We are building a barrier against infection. This means we cannot just use standard off-the-shelf components. We have to look at the entire system, from the intake valve to the dispensing nozzle, and ask: "Is this sterile?"
Potable water is free from all bacteria.False
Potable water meets safety standards but may still contain low levels of non-harmful bacteria.
Vulnerable groups require higher purity standards.True
Infants and immunocompromised individuals cannot handle pathogens that healthy adults can ignore.
2. Criterion #1: The "Log Reduction" Standard (99.999% Purity)?
Percentages on packaging can be misleading. A claim of "99% effective" sounds good, but in microbiology, it is actually a failure. We need to look at the math.
Medical-grade requires a "Log Reduction" standard. A 6-Log reduction means 99.9999% of bacteria are killed. This mathematical certainty separates professional sterilization from consumer-grade filtration, ensuring that out of a million bacteria, less than one survives.
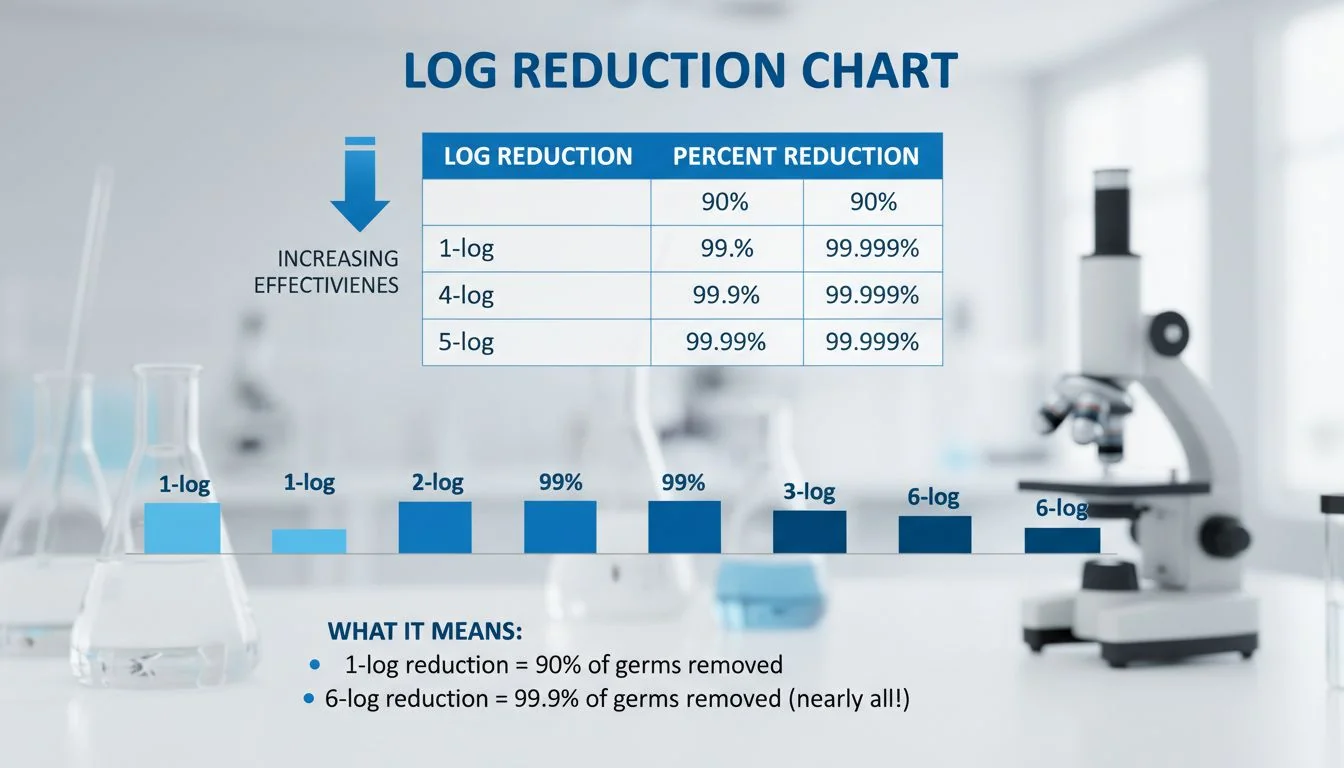
As engineers and designers, we love numbers. In the medical water industry, the most important number is the "Log." You should teach your B2B clients to use this terminology. It makes them sound like experts rather than just salespeople.
Here is how the math works. If you have 1,000,000 bacteria in a sample:
- 1-Log (90% reduction): 100,000 bacteria survive.
- 2-Log (99% reduction): 10,000 bacteria survive.
- 3-Log (99.9% reduction): 1,000 bacteria survive.
- 4-Log (99.99% reduction): 100 bacteria survive.
- 6-Log (99.9999% reduction): 1 bacteria survives.
See the difference? A standard consumer filter might claim 99.9% removal. That sounds great, but it leaves 1,000 potential pathogens in the water. For a healthy person, the stomach acid handles this. For a cancer patient or a newborn, this is a risk.
The Engineering Challenge
Achieving 6-Log reduction is hard. It usually requires a combination of technologies. You might use Reverse Osmosis (RO) followed by UV-C sterilization. As a designer, you have to fit these components into a compact chassis without compromising flow rate. It is a balancing act between kill-rate and user experience.
99.9% removal is sufficient for medical grade.False
Medical grade typically requires 99.9999% (6-Log) reduction to ensure safety for vulnerable users.
Log reduction measures the number of live bacteria remaining.True
It is a mathematical representation of the percentage of pathogens killed or removed.
3. Criterion #2: The "Sterile Pathway" (Material Science)?
The filter might work perfectly, but what about the pipes? If the water travels through dirty tubes, the filter is useless. This is where material science matters.
The "Sterile Pathway" ensures water touches only biofilm-resistant materials after filtration. This usually means replacing porous plastics with 304 or 316 Surgical-Grade Stainless Steel4 to prevent bacterial adhesion and ensure the water remains pure until it hits the glass.

This is my strongest technical argument, and it relates directly to mold design. We know that plastic injection molded parts have a surface texture. Even if it looks smooth to the eye, under a microscope, it has peaks and valleys.
These valleys are where "Biofilm" lives. Biofilm is a slimy layer of bacteria that sticks to surfaces. Once it forms on plastic, it is almost impossible to remove. It protects the bacteria from chemicals and UV light. In a hospital, biofilm is the enemy.
The Steel Advantage
This is why we must advocate for "Surgical-Grade Stainless Steel" (316) or "Medical-Grade Steel" (304).
- Plastic: Hydrophobic and porous. Bacteria stick to it easily.
- Stainless Steel: Hydrophilic and non-porous. It is much harder for biofilm to anchor itself.
When you design a medical-grade purifier, the post-filter tubing, the storage tank, and the faucet should be steel. This creates a "Halo Effect" for the product. It looks like a surgical tool. It feels cold and clean. It justifies a higher price point. If you can prove that Plastic = Biofilm Risk and Steel = Biofilm Resistant, you disqualify 99% of the cheap competitors.
Biofilm forms easily on smooth stainless steel.False
Stainless steel is resistant to biofilm formation compared to porous plastic surfaces.
316 Stainless Steel is considered surgical grade.True
It contains molybdenum, making it highly resistant to corrosion and suitable for medical environments.
4. Criterion #3: Prevention of Re-Contamination (The Closed Loop)?
Cleaning the water is the easy part. Keeping it clean is the hard part. Bacteria are in the air all around us, waiting to get back in.
A medical-grade system uses a closed-loop design. This prevents airborne pathogens from entering the storage tank, ensuring the water remains sterile from the filter to the dispensing point. Open tanks or jugs are immediate failure points.
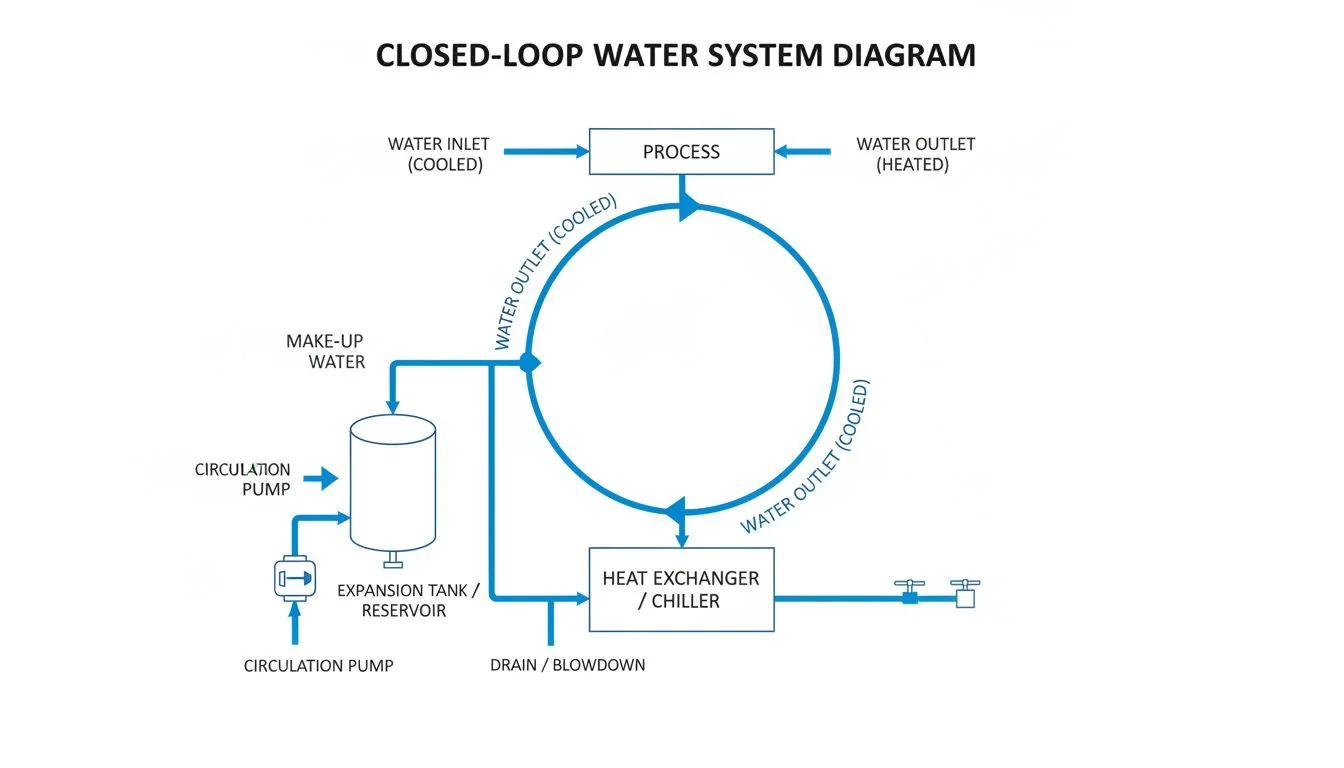
I have seen many "clean" water systems that use a simple gravity tank. The user lifts a lid to pour water in, or the tank has a vent hole to let air in so water can flow out. From an engineering standpoint, this is a disaster for sterility.
Every time air enters the tank, dust, mold spores, and bacteria enter with it. If the water sits in that tank for six hours, the bacteria multiply. The water you drink is no longer the water that passed through the filter.
The Design Solution
To solve this, we need a "Closed Loop."
- Sealed Tanks: The storage tank must be airtight.
- Air Filtration: If air must enter to displace water, it must pass through a HEPA filter first.
- Positive Pressure: Some advanced systems keep the tank under slight pressure so nothing can leak in.
- UV Recirculation: The best systems circulate the stored water past a UV lamp periodically.
This is complex to design. It adds cost. But for a vulnerable family, it is necessary. It ensures that the water in the glass is exactly as pure as the water leaving the membrane.
Air vents in water tanks are harmless.False
Unfiltered air vents allow airborne pathogens and mold spores to contaminate the stored water.
UV recirculation helps maintain sterility in storage.True
Periodic UV exposure kills any bacteria that might try to grow in the storage tank.
5. Criterion #4: Certified Validation (No "Trust Me")?
In this industry, anyone can print a gold sticker that says "Best Quality." Real safety requires proof, not just marketing promises.
True medical-grade devices rely on Certified Validation5 from independent labs. They do not rely on internal testing but prove their claims through rigorous, standardized protocols like NSF/ANSI 55 Class A, providing data sheets that verify performance.

When I source molds or CNC parts, I do not just take the supplier's word for it. I ask for the inspection report. I want to see the dimensions measured. The same applies here.
There is a big difference between "Tested to NSF Standards" and "NSF Certified."
- Tested to: The manufacturer did the test themselves, or paid a lab to run a single test. They might have passed once.
- Certified: The agency (like NSF or WQA) inspects the factory, tests the product regularly, and ensures consistency.
The "Med-Tech" Partner
This is where you shift your positioning. You are not an appliance manufacturer. You are a "Med-Tech Engineering Partner." You build systems that meet clinical standards. This allows you to sell to Dentists, Clinics, and Luxury Spas. These clients need paperwork. They need liability protection. By providing certified validation, you give them peace of mind. You are selling certainty, not just a machine.
Internal testing is sufficient for medical claims.False
Internal testing lacks the objectivity and rigor of third-party certification required for medical devices.
Certification involves ongoing factory inspections.True
Reputable certifiers check the manufacturing process regularly to ensure consistent quality.
6. Conclusion: Elevate Your Brand to the Clinic Standard?
You are not just making a machine. You are building a health solution. The market is crowded with gadgets, but empty of true solutions.
By adopting these standards—Log Reduction, Surgical Steel, Closed Loops, and Certification—you move from selling appliances to providing Med-Tech solutions. This opens doors to high-value markets like clinics and health-conscious families who need absolute reliability.

We have covered a lot of ground. We moved from the math of Log Reduction to the material science of stainless steel. We looked at the engineering of closed loops and the importance of validation.
For a designer like Jacky, this is an opportunity. It is a chance to design something that matters. It is not just about making a plastic shell look pretty. It is about integrating high-grade materials and precise engineering to save lives.
When you position your product as "Medical-Grade," you are making a promise. You are telling the mother mixing formula that she is safe. You are telling the dental surgeon that his equipment is sterile. To keep that promise, you must respect the engineering. You must fight biofilm with steel. You must fight bacteria with math.
If you do this, you are no longer competing on price. You are competing on trust. And in the medical world, trust is the most valuable currency of all.
Medical-grade positioning allows for higher price points.True
The increased safety and material quality justify a premium price for high-intent buyers.
Design aesthetics are more important than material choice.False
In medical devices, material choice (like steel vs plastic) is critical for function and safety.
Conclusion
To build a true medical-grade purifier, you must combine 6-Log sterilization, biofilm-resistant stainless steel, closed-loop engineering, and third-party certification. This elevates your product from a kitchen appliance to a trusted health necessity.
References
-
Understanding Log Reduction is crucial for ensuring the effectiveness of water purification systems, especially for vulnerable populations. ↩
-
Understanding engineering standards helps in designing effective and safe medical-grade water purification systems. ↩
-
Medical-grade water must meet strict standards for purity, making it essential for immunocompromised individuals. ↩
-
Surgical-Grade Stainless Steel is essential for preventing biofilm formation, ensuring the purity of medical-grade water systems. ↩
-
Certified validation ensures that medical devices meet rigorous safety standards, providing peace of mind to users. ↩